
For example, in the MFS case, protonation of the transporter applies an extra electrostatic force, which together with the hydrophobic mismatch forces generates a torque to induce conformational changes for substrate transport. This hypothesis has been used to explain the energy coupling mechanisms of PMF-driven major facilitator superfamily (MFS) transporters (Zhang et al., 2015a), of proton transfer-mediated GPCR activation (Zhang et al., 2014), and of the ABC transporters (Zhang et al., 2015b). The central idea of this hypothesis is that the interaction of charged groups in a membrane protein with ΔΨ can provide a major driving force for the functional cycle of the corresponding membrane protein. Recently, we proposed an electrostatic membrane potential (ΔΨ)-driving hypothesis in an attempt to unify energy-coupling mechanisms of a variety of transmembrane proteins into a common theoretical framework. Although several models of converting the electrochemical potential energy of protons into the rotational kinetic energy of the F o rotor have been proposed, a consensus remains to be established. While the F 1 mechanism has been well established (Boyer, 1993), mechanisms of the F o motor remain controversial. More importantly, each member of the ATP-synthase family contains two motor sectors: F o couples proton translocation to rotation, and F 1 couples rotation to ATP synthesis or hydrolysis (Fig. Therefore, for simplicity, here we will focus on PMF-driven ATP synthases. Moreover, some rotors of ATP synthases even switch between PMF and Na + as their driving substance depending on the supply of either of these two types of ions. Because of the homology of the binding sites for proton and Na + (Gruber et al., 2014), the same principles of energy transfer of proton motive force (PMF)-driven ATP synthases is likely to be applicable to Na +-driven ATP synthases as well. As implied by Peter Mitchell’s chemiosmotics theory developed in the 1960s, ion-translocating rotary ATPases serve either as ATP synthases, consuming energy supplied by transmembrane ion motive force (mostly from H + or Na +) to generate ATP, or as transmembrane ion pumps powered by ATP hydrolysis.
Enzymes of this family catalyze either ATP synthesis or its reverse reaction, ATP hydrolysis.

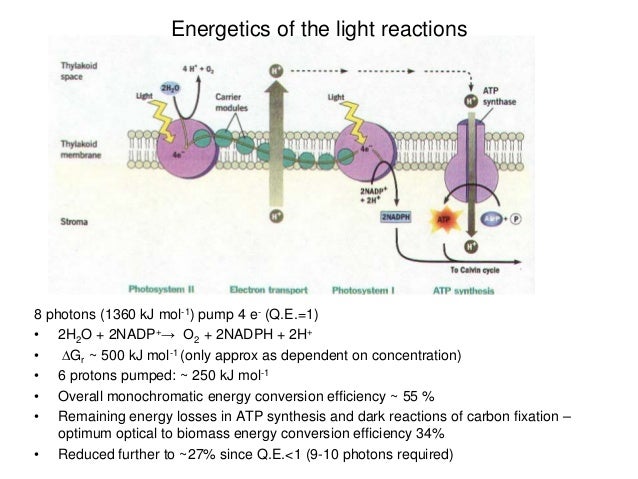
Members of the ATP synthase family function as nano-turbines, possibly the smallest that exist in nature. The complexes of triphosphate adenosine (ATP) synthases belong to one of the most ancient protein families, and as such the complexes are ubiquitously distributed in all life kingdoms (Gruber et al., 2014 Nakamoto et al., 2008). This hypothesis offers new directions of combining experimental observations with thermodynamics and is a step further towards understanding the mechanism of the rotation of the F o motor. In this hypothesis, the shape difference between the interface-located trajectories in the rotator- and stator-reference systems ensures the unidirectional rotation of the F o motor.

#Proton movement across membrane drives rotation of c ring free
In this article, based on theoretical considerations and in light of recent structural insights, Zhang and colleagues propose that the F o rotation is driven by a noise-resistant thermodynamic process which converts the electrostatic free energy of the cations moving in the rotor-stator interface into the rotational kinetic energy.
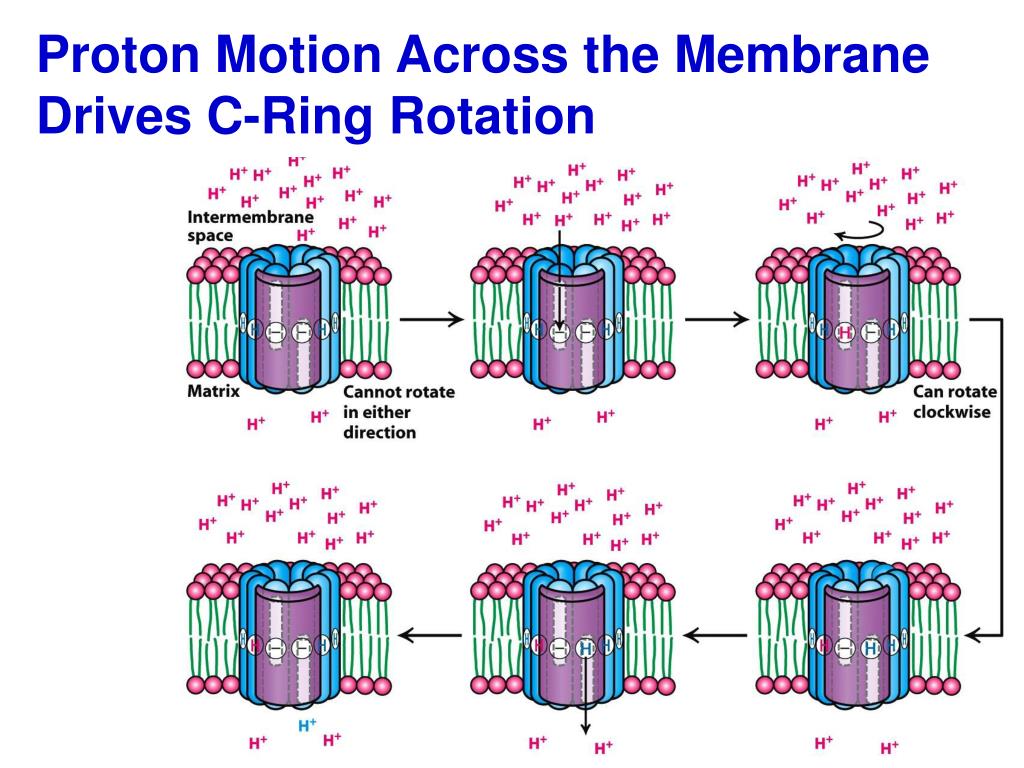
An essential step of this process is to utilize the free energy of the transmembrane electrochemical potential of cations, such as protons or sodium ions, to drive the rotation of the F o motor.Īlthough membrane potential has been proposed to fuel the process for over ten years, this thermodynamic concept has not been firmly accepted in the ATP synthase research field partially because of lack of detailed mechanisms. A REVISIT TO AN ANCIENT MOLECULAR MACHINEĪTP synthesis via F 1/F o-like ATP synthases is a universal energy conversion process in all living cells.


 0 kommentar(er)
0 kommentar(er)
